What is electrolyzed water?
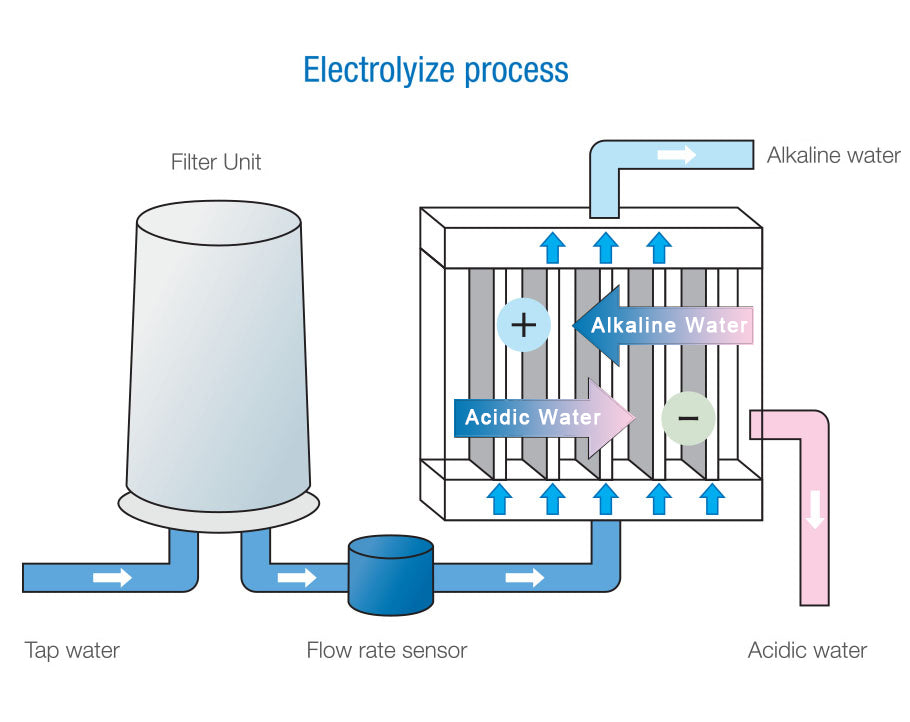

Electrolyzed water is water produced by electrolyzing water.
Electrolytic water is usually the product of electrolysis of water containing salt (e.g. sodium sulfate, not table salt, which produces chlorine gas). After electrolysis, the water itself is neutral, and other ions can be added, or it can be separated by a semi-permeable membrane to produce two types of water. One is alkaline ionized water and the other is acidic ionized water. Electrolytic water with sodium chloride as the electrolyte in the water will contain sodium hydroxide, hypochlorous acid and sodium hypochlorite after electrolysis (if pure water is electrolyzed, only hydroxide ions, hydrogen gas, oxygen and hydrogen ions will be produced).
It is well known that hydrogen and oxygen are generated by passing electricity through water. When water is electrolyzed, hydrogen is generated by the progress of the reduction reaction at the cathode, and alkaline ionized water is generated. On the other hand, at the anode, oxygen is generated and acidic ionized water is generated. In other words, this alkaline ionized water and acidic ionized water produced by electrolyzing water are electrolyzed water.
It is well known that hydrogen and oxygen are generated by passing electricity through water. When water is electrolyzed, hydrogen is generated by the progress of the reduction reaction at the cathode, and alkaline ionized water is generated. On the other hand, at the anode, oxygen is generated and acidic ionized water is generated. In other words, this alkaline ionized water and acidic ionized water produced by electrolyzing water are electrolyzed water.
Under certain conditions, the acidic electrolytic water produced after electrolysis is used for sterilization. Oxygen generated at the electrode according to the electrolysis principle, at low pH (e.g., pH < 2.7), will combine with chlorination to produce an aqueous solution of hypochlorite or chlorite ions.
In addition, although some advertisements claim that alkaline electrolytic water has the function of "neutralizing acidity," the alkaline water produced by electrolysis is actually turned into acid when it reaches the stomach by the strongly acidic stomach acid.
In addition, although some advertisements claim that alkaline electrolytic water has the function of "neutralizing acidity," the alkaline water produced by electrolysis is actually turned into acid when it reaches the stomach by the strongly acidic stomach acid.




